The Bugs We Live With - And a Look at My Gut Microbiome
I’m a scientist involved in the human microbiome project, which studies how changes in the normal bacteria present on our bodies affect human disease. Today’s post is an overview of the human microbiome and a personal look at the bacteria in my gut.
The Human Microbiome
The bacteria that live in our gut, on our skin, in our lungs and elsewhere in our bodies influences our susceptibility to disease. There are thousands of different bacterial species within a single person. Luckily, most bacteria are not harmful, and many of them actually play a beneficial role. Together, these communities of bacteria make up the human microbiome.
Every body site has a distinct bacterial community. Characterized by different properties, each body site requires a different set of bacterial species. Not only do the bacterial communities associated with various body sites differ, they also differ among individuals. Even differences in your genes can influence bacterial populations, affecting things like the acidity of the digestive tract, or the robustness of an immune response.
Beginning at birth
Although bacteria have been found in placenta, the main colonization of an infant’s body occurs during birth and mode of delivery has been shown to play an important role in this process. Vaginally born infants’ skin contains Lactobacillus, Prevotella or Sneathia species very similar to the species found in their mother’s vagina, whereas Cesarean section born infants harbor bacteria similar to those found on their mother’s skin. The differences among these infants’ microbiomes can last for months, and some studies suggest C-section children are at higher risk for asthma, obesity and other metabolic diseases later in life. Interestingly, although biological mothers seed their infants initial microbiome, one study showed that the gut microbiota of teenage twins in the US were no more similar to their mothers than to their fathers. In addition, genetically unrelated but co-habitating mothers and fathers had bacterial communities that were significantly more similar than were members of different families. But microbiome research is still a young field, and it is much too early to claim that we know the long term impact of these differences.
When bacteria go amok
As we learn more about the bacteria that keep us healthy, we are also gaining a better understanding how subtle imbalances can lead to adverse health outcomes. Acne, for example, is an irritating, although not particularly dangerous, skin condition. At the root of acne is an imbalance between acne-causing bacteria and healthy bacteria. The relationship between adverse health outcomes and the microbiome is typically more complex. Consider obesity, a highly heritable condition, that is also clearly influenced by sedentary lifestyle and poor food choices. Several studies have demonstrated that thin people and obese people have different bacterial communities in their guts. And when obese people lose weight, their bacterial communities change accordingly.
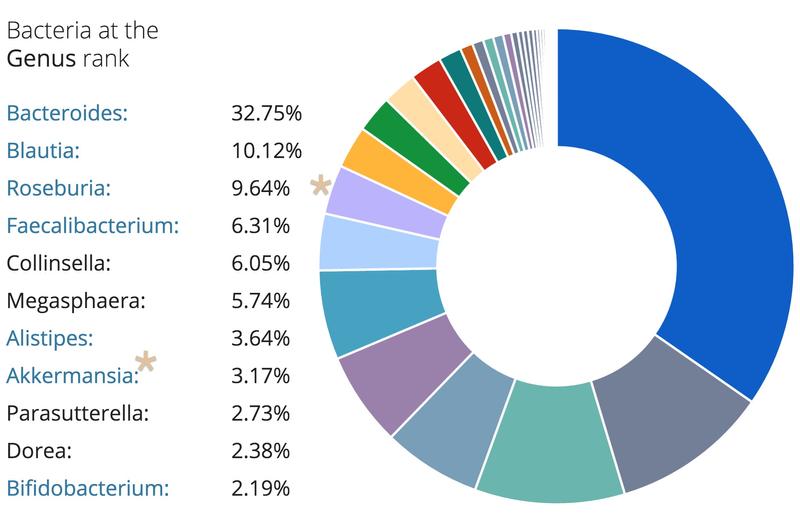
A peek into my gut
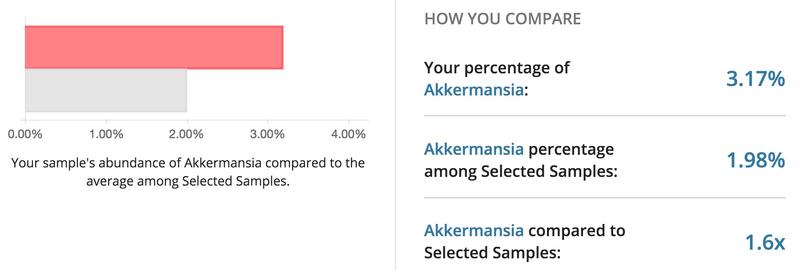
Using a commercially available kit from uBiome, I had the bacteria in my gut sequenced. Below are the top bacteria found in my sample. Interestingly, I had a higher percentage of Akkermansia compared to the average participant in the uBiome database. Recently, Akkermansia has been shown to prevent weight gain and inflammation. My gut microbiome was also shown to be dominated by Bacteroidetes, which is correlated with weight loss and lower body weight. It will be interesting to see if the percentage of Akkermansia and dominance of Bacteroidetes stays constant as I get older or if I will see a decrease over time.
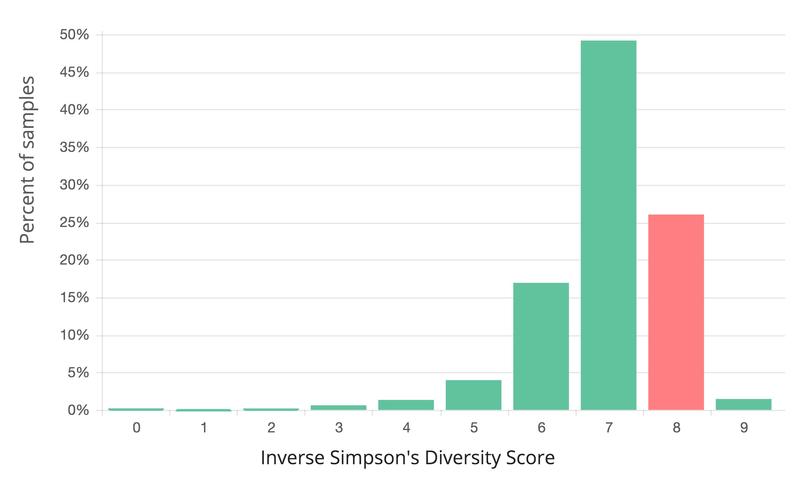
Greater microbiome diversity is associated with health, but varies among individuals. When compared to samples of the same type in the uBiome database, my gut sample was 76% more diverse than all other gut samples.
Are we too clean?
Despite an increasing awareness of the benefits offered by bacteria, many people still obsessively avoid germs. We hover above public toilets, wipe down grocery cart handles, press elevator buttons with our knuckles and disinfect all our kids toys. We also constantly wash our skin with antibacterial soaps and wipe out our gut bacteria when we take antibiotics. The “hygiene hypothesis” suggests that a lack of exposure to microbial stimulus early in childhood is a major factor involved in the development of allergic reactions and immune-related disorders.
While it’s true that our lives are undeniably better since the discovery of antibiotics, many other diseases such as allergies, inflammatory bowel syndrome, asthma, diabetes and obesity are on the rise. Given that our microbiome is important in maintaining a healthy state, the increased use of antibiotics combined with the reduced exposure to bacteria might be related to the increase in diseases of the immune system and obesity. Obviously, we should still support vaccinations and we should still treat infections with antibiotics, but we should also embrace that a healthy and diverse microbiome is important for our health.
How to sequence YOUR microbiome
If you want to get your microbiome sequenced, use this code to get 15% off your first uBiome kit (think Christmas/Hanukah gift for the science geek in your life!). Images from this post are from the ubiome participant interface.
